3 min read
Perfecting the Platform
Elosity is a research-driven product designed specifically to address the needs of pharmaceutical and clinical research organizations. Over three and a half years, I collaborated with a team of product strategists, managers, researchers, and developers to bring Elosity from concept to launch. My contributions included shaping the strategic vision, defining the product roadmap, and designing the user experience and its underlying design system.
This project aimed to modernize how pharmaceutical studies are conducted. Before this, clinical trials relied heavily on manual processes, like paper-based data collection, which were prone to errors. Our work was part of a larger shift, starting around 2009 with the introduction of enterprise software for pharmaceutical research, and accelerating by 2020. This transformation, fueled by advancements in cloud computing, artificial intelligence, and digital tools, addressed the growing need for efficient and reliable clinical trials.
During our research for the clinical trial platform, we identified a diverse range of users, each with unique needs and challenges. These personas included: Investigators, who needed efficient tools for data collection and patient management; Patients, who required clear communication and easy access to trial information; Suppliers, who managed the logistics of trial materials; Sponsors, who oversaw the overall progress and compliance of the trial; Managers, who coordinated resources and workflows; and Designers, who ensured the platform was user-friendly and accessible, and met the requirements of the protocols. Uncovering the specific needs of each persona was crucial for building a platform that truly served everyone involved in the clinical trial process.
Design by Accretion
Using evaluative AI through Figma’s platform, I collated all feedback from 3 primary data collection efforts. From Product-Market fit questionnaires, first-hand research interviews, and from backlog feature request: I drew insights and product direction based on some of the summative and evaluation that AI provided, only as an informer on our codification of the data. After iterating with key stakeholders for three cycles, we developed a shared vision of the problem we were solving and the design to address that problem.
Our high level goals were to:
Make it fast and easy to use for everyone, everywhere.
Give users more control over their time and money.
Create a platform for innovation and deeper engagement.
One of the most significant design challenges was handling blinded information. In clinical trials, accidental disclosure of this data can invalidate the entire study, leading to costly restarts. Our research, including contextual inquiries with clinical trial managers, revealed a need for a clear, unobtrusive way to protect this information. We developed a solution that used a 'key' UI element to obfuscate sensitive data. Working closely with the development team, we implemented this using a lightweight Unicode key, effectively securing blinded information without hindering usability.
User Flow and artifacts for Reports experience.
Onsite Clinician User Experience for Reports
Study Designer experience for creating reports.
My Role
I led the design of the clinical design and live-study experience between November 2020 and August 2024 and collaborated with two other designers on the Home experience, Design-Trial experience and Live-study experience and features.
In addition, I hired and worked alongside a Researcher, Prototyper, Content Strategist and 4 Product Managers.
I stopped working on the project during the latest delivery phase as the app started to be sent to market, due to a Private Equity acquistion.
The app launched globally on August 31st, 2024.
The Challenge
High Quality for everyone, everywhere
Many individuals are excluded from clinical trials due to geographic limitations. Direct-to-patient experiences offer a solution by expanding participation to a wider population. We designed a comprehensive caregiver and patient experience to address this. The core challenge we tackled was simplifying the complex process of a patient visit for individuals without medical expertise. By streamlining steps and removing unnecessary information, we made clinical trial participation more accessible and manageable for non-medical caregivers and patients.
Kickoff
Picking up the pieces
Initially, the project lacked a defined mission and specific goals for the platform experience. We were starting with a blank slate. To address this, and without any existing user insights, I collaborated with our researcher, Lakisha, to conduct exploratory research. Our aim was to understand how clinical trial operators and patients were currently interacting with the existing processes and systems.
We designed a patient experience that prioritizes convenience and accessibility for at-home clinical studies. This included enabling remote doctor access, allowing patients to participate in trials from the comfort of their own homes. We focused on streamlining the process, providing clear instructions, and ensuring seamless communication with healthcare providers. This approach aims to reduce the burden on patients, improve participation rates, and expand access to clinical research for individuals in remote or underserved areas.
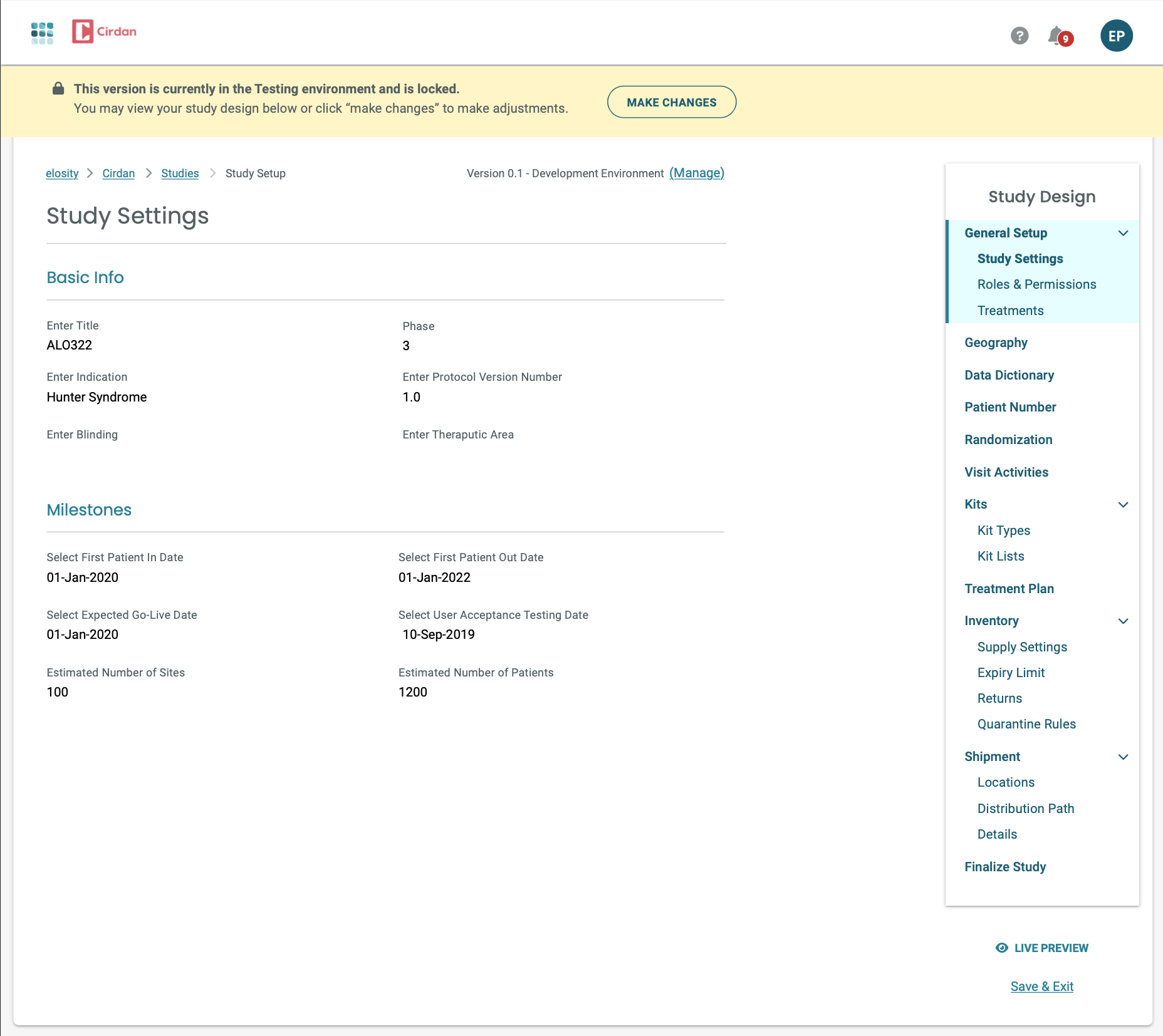
We encountered a significant challenge: study designs needed to be updated while active studies were in progress, with patients already receiving treatment. This required a robust versioning system. To address this, I designed and built a version control feature, allowing for seamless study amendments. We began by conducting in-depth user research with Study Designers, understanding their specific needs. Our goal was to create an intuitive experience that enabled them to easily implement study design changes—driven by scientific discovery or early study data—without disrupting the ongoing trial.
To visualize the user flow, we first created low-fidelity sketches for accuracy and stakeholder review. We then utilized our design system, HelixDS, to quickly develop prototypes and detailed screen specifications, effectively showcasing the user experience's visual design and interaction.
We meticulously documented screen specifications and designs for every stage of the versioning process. This included detailed layouts and interactions tailored to the specific needs of each user role involved, illustrating how they would accomplish their respective 'Jobs To Be Done' within the system.
Deeper Insights
Working backwards from Perfect
This project was a massive undertaking, divided into three core areas: Core functionality, Study Runtime operations, and Study Design. Even in its initial release, it encompassed hundreds of distinct functions and intricate user journeys. To give you a sense of scale, this release was just the first of eight planned releases, all contributing to the overarching Product Vision and Roadmap. We were building a truly comprehensive platform.
General Mall Map for the entirety of the minimum lovable product.
The brand identity, marketing collateral, and go-to-market (GTM) strategy were strategically developed through a coordinated effort between an external agency and our internal marketing team. This included comprehensive market analysis, audience segmentation, and the creation of targeted messaging and materials to support a successful product launch.
LabCorp Endpoint Clinical | Elosity Platform
Coverage & Awards: Acquired for $500m. Launched first of its kind platform for clinical trial management.
Role: Design Principal, Head of Product Experience Design & Research
Methods: Contextual Inquiry, User Research, Journey Mapping, Design Thinking, Competitive Analysis, and Market Research,
Artifacts: Discovery Read-outs, Journey Maps, Personas, Wireframes, Clickable Prototypes, Helix Design System, REACT App, Accessibility/WCAG adherence, Responsive Design Specifications, Product/Application itself, and Brand Creation.
Created at LabCorp Endpoint Clinical.